Organovo: bioprinting tissue to speed up drug development
Organovo, kicked off use of 3D printed liver tissue in big pharma research and drug development. Can it catapult the industry to shorten drug development cycle time and print entire liver organs in the next 15 yrs?
Bioprinting- “the liveliest—literally—field of 3D printing may sound like something from a sci-fi movie, but (spoilers) it’s real and happening now” [1]. While technology is still in its early days of the ability to print entire organs, significant advances have been made in the ability to bioprint tissues and vascular structures (blood vessels).
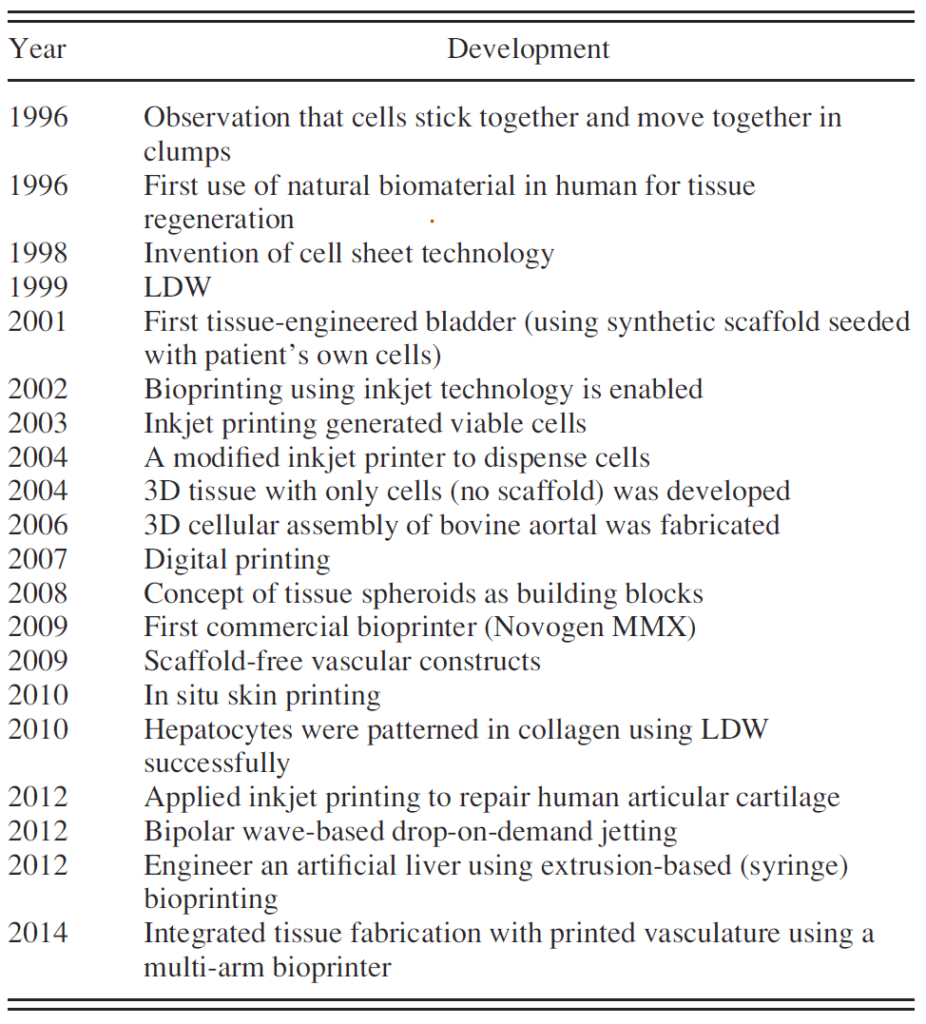
In fact, Organovo invented the world’s first commercially available bioprinter Novogen MMX in 2009 which gained traction with academic and research labs (see Figure 1) [2]. In big pharma drug development, bioprinting has remained a more distant reality up until the last few years. Organovo is changing this and has started to set up partnerships on development of bioprinted tissue with at least 6-10 major pharma companies, and most publicly with Merck in 2015, creating the opportunity to accelerate drug development cycles [3, 4].
What exactly is the Organovo technology
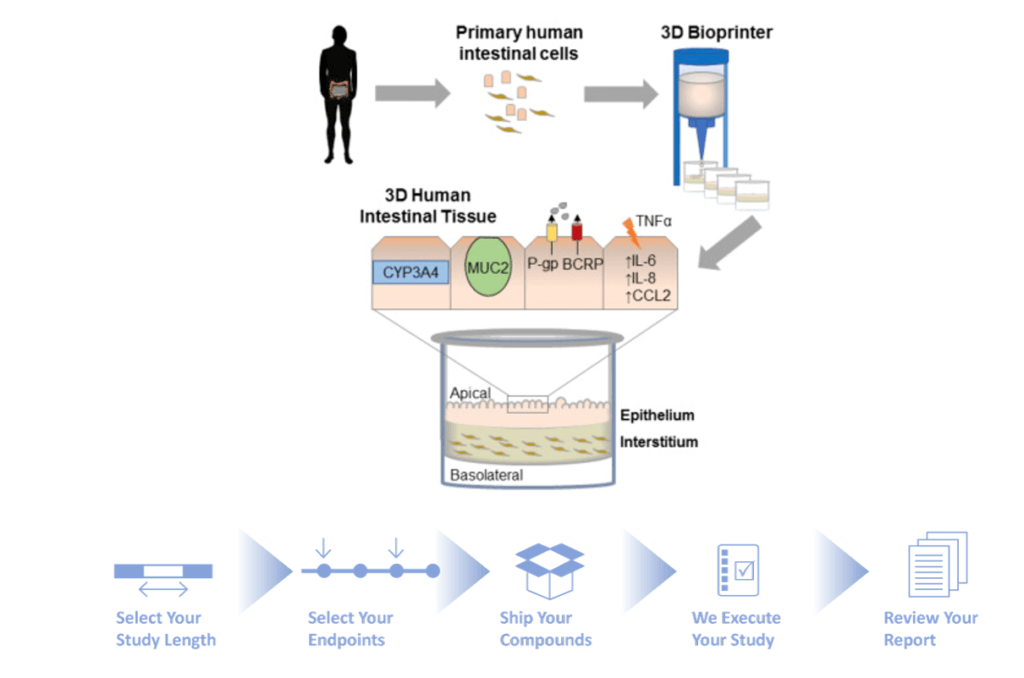
Organovo offers customers (pharma companies and academic research facilities) “structurally and functionally accurate bioprinted human tissue models”, specializing in the liver [5]. Much like traditional 3D printing, the NovoGen Bioprinter® Platform (MMX-07) does “inkjet bio-printing” – using “bioink” to print living cells layer-by-layer [2]. Besides manufacturing the printer, Organovo sources normal or diseased human cells from its subsidiary Samsara and produces its own NovoGel® Bio-Ink to use as inputs into the printer platform. The bioprinted tissue output is used in predictive preclinical testing of drug compounds that is either performed by Organovo itself or the customer. See Figure 2.
Why do we care?
- More and better quality of drugs (with efficient toxicity studies) – Merck and Organovo hit their first key development milestone of their research partnership in April 2018, publishing scientific evidence that shows 3D printed tissue is better than 2D alternatives used in traditional pre-clinical testing [6]. Higher quality of testing using readily available bioprinted tissue means improved drugs and more drugs passing through the development process.
- Shorter drug development cycle – Liver toxicity is the #1 reason pharma companies discard drug trials. Efficient toxicity studies using Organovo’s bioprinted tissue means disease evolution can be assessed in a more true-to-life way, and a go/no-go decision can be made sooner in the drug development process [7]. Further, by collaborating with pharma companies directly on research, the lag time of relevant research reaching industry-insiders is shortened.
Opportunities on the horizon
In the short term, Organovo has already joined the Advanced Regenerative Manufacturing Institute (ARMI) ecosystem which has 100 partners. ARMI has over $300M in funding and provides the opportunity to collaborate with others to engineer scalable manufacturing for bioprinted tissues [8].
In the long term, Organovo is approaching the FDA in early 2020 with an investigative new drug (IND) application wherein its bioprinted liver tissue can be used for the treatment of rare diseases [9]. CEO Keith Murphy and senior management has committed progress to the street in multiple earnings calls towards filing an IND in 2020 for orphan designation [10].
Longer term challenges and recommendations
- Profitability: Organovo is one of the only publicly listed companies in the bioprinting industry and withstands constant scrutiny from the market. It has been unprofitable in the last few years, with falling share prices and will run into further cash flow problems if it needs to accelerate R&D to meet its 2020 targets. My (arguably) radical recommendation would be to consider taking the company private for a few years and raising cheaper capital from investors looking for long term returns.
- Technical hurdles: Significant technical challenges still exist with bioprinting around sensitivities of living cells and the construction of tissues. My recommendation would be to embrace open innovation (in the ARMI ecosystem or otherwise) and offer multiple academic institutions the opportunity to rapidly test Novogen bioprinting platform compatibility with more kinds of bioinks, biomaterials and thus more use cases simultaneously.
- Competition: Several other startups are vying to achieve scalability in the space [2, 11]. My recommendation would be to learn from their competitors’ business models. For example, Advanced Life Solutions’s approach is to provide an end-to end solution. It sells its BioAssembly bot (6-arm robotic printer) printer directly to customer but makes profit from its bio-inks and pre-processing software technology that allows researchers to own the modeling of the human tissue [12].
Questions remaining in the Petri Dish
- Approximately 1500 patients on the waitlist for liver transplants died in 2016 before they could receive an organ [13]. How can Organovo build on competitor offerings in the bioprinting industry to save lives by printing new livers on demand?
- What are the ethical concerns as Organovo aggressively approaches the FDA for the IND (Investigational New Drug) orphan drug status? Should bio-printed tissue / organs be regulated as biological products or drugs/devices?
(790 words)
Bibliography
[1] Sirinterlikci, Arif, and Lauren Walk. 2014. “Bioprinting: Science or Fiction?“, SME 2014 Medical Manufacturing Yearbook of Manufacturing Engineering
[2] Dababneh, Amer B., and Ibrahim T. Ozbolat. 2014. “Bioprinting Technology: A Current State-Of-The-Art Review”. Journal Of Manufacturing Science And Engineering 136 (6): 061016. doi:10.1115/1.4028512.
[3] Organovo. April 22, 2015 Form 8-K,<https://www.sec.gov/Archives/edgar/data/1497253/000156459015002746/onvo-8k_20150421.htm>, accessed November 2018.
[4] Speights, Keith. 2018. “5 Things Organovo Holdings, Inc. Management Wants You To Know — The Motley Fool”. The Motley Fool. https://www.fool.com/investing/2016/06/13/5-things-organovo-holdings-inc-management-wants-yo.aspx.
[5] Organovo website
[6] Madden, Lauran R., Nguyen, Theresa V. et. al. “Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions”. iScience. Volume 2, 2018, Pages 156-167, ISSN 2589-0042, https://doi.org/10.1016/j.isci.2018.03.015.
(http://www.sciencedirect.com/science/article/pii/S2589004218300245)
[7] “Better Tests For Liver Toxicity Would Mean More Medicines — And Safer Medicines — For Patients – American Chemical Society”. 2018. American Chemical Society. https://www.acs.org/content/acs/en/pressroom/newsreleases/2013/september/better-tests-for-liver-toxicity-would-mean-more-medicines-and-safer-medicines-for-patients.html.
[8] Organovo. Organovo and Samsara Sciences Partner with New Manufacturing USA Institute. [online] GlobeNewswire News Room. Available at: https://globenewswire.com/news-release/2018/05/03/1495905/0/en/Organovo-and-Samsara-Sciences-Partner-with-New-Manufacturing-USA-Institute.html [Accessed 13 Nov. 2018].
[9] Organovo. Investor Presentation March 2018. Available at Capital IQ.
[10] Organovo Holdings, Inc. NasdaqGM:ONVO. FQ2 2019 Earnings Call Transcripts. Thursday, November 08, 2018 10:00 PM GMT . Available at Capital IQ.
[11] The Economist. “A Tissue of Truths – Printed human body parts could soon be available for transplant” Jan 28th 2017.
[12] Peels, Joris. 3Dprint.com “Interview with Jay Hoying and Michael Golway of Bioprinting Company Advanced Solutions Life Sciences” November 7, 2018. [Accessed 13 Nov. 2018]
[13] Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2016 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2017 http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx Accessed 12th November 2018



It is incredible that 3D printing has advanced to the level of printing layers of living cells. Along with improvements to the drug development method as you mentioned, the social implications of printing organs for testing are expansive—such as eliminating the need to test products on animals. It would be interesting to know the cost of printing these organs currently, and the what timeline exists to reduce production costs to a level where the printing method could be more widely used. Another question to consider is whether there are any logistical or ethical issues around sourcing cells that would be turned into printing material? It would also be interesting to see what measures Organovo is taking to collaborate with other startups that are developing this technology.
Interesting to hear about Organovo using bioprinting with a unique commercial strategy of partnering with pharma companies who will benefit in the drug development cycle. I would imagine that 3D printing the liver will have high upfront costs, but the experience and technology has the potential to be transferable to other organs. I have heard of other companies working on using 3D printing to create scaffolding tissue used in the heart. Perhaps Organovo’s approach will be less of an upfront challenge and lead to the company’s eventually ability to accomplish such a feat. I hope that Organovo can deal with the public pressure and maintain a long term view to accomplish this!
I really enjoyed reading this – super cool/interesting that there are bioprinting companies that are creating real body parts. I’m hopeful that Organovo is able to overcome there cash flow issues as it would be a shame for a company that is working on such an important/vital societal issue to go bankrupt. I guess I am curious what other body parts Organovo has in their pipeline? I am also curious if Organovo believes it will be possible to eventually print a human and in what time frame? I thought this was super intriguing and I look forward to continuing to follow developments in this space!
I agree with the above commentary, this is a very interesting topic. I recently attended a panel on “Human Augmentation” as part of the tech conference and one of the topics discussed on the panel was exactly this, the use of 3D printing of tissues. One of the speakers is the co-founder of ApreX Biotech, which uses origami to create artificially enhanced organ tissues for drug development and transplantation. He talked a lot about how this technology can help drastically reduce the development costs of drugs, the need for animal testing and the potential development of more customized medical solutions. I too this this technology has so much potential on a global scale. I do worry that if this technology does become more readily available will people take worse care of their bodies knowing they can replace organs as necessary?
This is truly a novel technology! I think Organovo is smart to use it for drug testing first before it can be used to replace human organ. I think FDA should definitely plays a more active role there to investigate the efficacy of such method of drug testing. After all, it is a simple gathering of cells without other organ systems.
This is incredibly interesting. It seems that this is something that would have many potential applications, so it is surprising that they are having trouble monetizing it. I really like the author’s suggestion to turn to open innovation to discover new applications of the technology.
Fascinating and great suggestions. I agree with everyone above that the potential of this technology sounds so high and the social benefit is so great that Organovo should consider open innovation to accelerate the development process.
Love the idea of making the drug development cycle faster to develop better drugs that can reach patients that can´t wait faster. One of the concerns I do have about the current process you describe is how the company can secure a constant supply of human cells to “feed” the printer (I guess partnerships with academic and medical institutions will become more crucial). My other concern is around regulation and if there exists a scenario in the future were this could replace human testing in the FDA drug development cycle. Overall, I think this technology is amazing and has a huge potential for the industry. Great essay!
Fascinating article – the progress in the field of regenerative medicine in the past few decades has been outstanding. 3-d bioprinting offers another step-change in the ability to bridge the gap between artificially engineered tissues and native constructs by virtue of the versatility to co-deliver cells and biomaterials with precise control over the end-state architecture.
As recently as 2014, the production of solid organs was considered a long-term aspiration, a challenge which Organovo appears to have tackled admirably (1). However, I would be eager to see more progress made with other solid organ types which have their own unique engineering challenges (such as the lack of the regenerative capacity of liver cells, amongst others).
1. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773.
Super interesting article and technology – we are at an exciting time in the development of regenerative medicine and therapies. As mentioned in the article, there is an interesting tension between control and speed as it comes to the innovation required to drive meaningful results in the business / industry – both from a profits standpoint and a patient outcomes standpoint. The article suggests moving to an open innovation paradigm, partnering with academic institutions and others. While this may increase the speed to market of an effective treatment / product, it seems like the value in this business is in the intellectual property. By moving to an open innovation paradigm, the company may ultimately lose control of the IP that makes the produce successful and therefore the ability to extract rents for that development.
That header image really freaks me out! 🙂 But that’s beside the point. This is a fascinating technology. I agree with your recommendation to take the company private. They should be focused on development & not share prices at this point
It’s mind boggling to see where technology in the health care sector is moving. Along with pre-clinical trials, I suspect the company is also thinking of ways where it can move upstream to a point where trials on humans can be minimized. I do wonder though, is replicating a certain tissue enough if we truly want to understand the interaction the drug will have with not just that tissue, but other parts of the body? I am far from an expert in Biology, but how much should we be thinking about this interactions element. Furthermore, how can we make this technology more cost efficient?
Interesting read! I am fascinated by the technological advances in 3D printing and the ability to completely transform the medical field. I agree with your suggestion to take the company private to raise capital. The technology they are creating is unbelievable and being subjected to market pressure may hurt the completion of the drug in a realistic time frame – which would be very disappointing.
This is phenomenal. This is just amazing technology. I would be curious to know how patients feel to accepting these printed organs even if FDA approved. Regarding going private, I would agree that it would create less of a distraction not having to see your stock price everyday, but that also being said in the private markets the capital raised would be more expensive not less which might not be something they can afford.
Fascinating stuff! Did you get a sense for what are the largest barriers to profitability? Are they the technical challenges or are they demand-based? A few years back, I attended on a talk on biomedical 3-D printing in which the company was using collagen protein to print organic structures — is Organovo utilizing this? Also, I’m really glad you brought up the ethical concerns question. It seems to me in the near term, it is a positive outcome to use Organovo product for testing instead of animals, however, as they and other companies become more proficient at creating tissue, we will certainly have to reckon with the implications of artificial biology. Homo Deus by Yuval Noah Harari examines these developments as well as the growth of artificial intelligence and what impact the two trends will have on different classes in society (the wealthy more-so in the near-term).
Thank you for the article Keeps you guessing. I think that the potential of this technology goes far behind what we can imagine today. Some questions that I get while reading this:
1. How different is it printing a single tissue organ, like a liver, from building a more complex part of the human body, like a limb.
2. Is there any risk of the body rejecting this type of tissues? From my understanding, they are made from the same DNA as the patient, so there should be no risk, right?
3. When combining this with bio engineering and genetics techniques, is it possible the create better human parts? For example, if a patient has certain heart condition, would it be possible to print a new heart for the person, modifying his DNA to solve the issue and then making a transplant? Or there would be any kind of rejection of the tissue because it’s a different DNA?
4. Will healthy people at some point decide to proactively replace certain organs or limbs, to get a “better” replacement? For example, an athlete that wants to get a higher performance, could decide to replace its lungs for ones that can offer him better resistance. I personally think that this is going to happen at some point, I just wonder how will society react.
Thanks for the article again, I really enjoyed it.
Bioprinting technology holds significant potential to transform the medical industry as well as to save lives, but I think it also raises some urgent ethical questions. My initial thought was that this could lead to a decrease in human/organ trafficking, but at the same time, I couldn’t help wondering if bioprinting would lead to a sort of ‘commodification’ of body parts, which would also be vulnerable to exploitation. Therefore I believe there is a strong need for strict regulations as a biological product.
Thanks for sharing. This is an amazing technology that I was thrilled to learn about. I’m curious about how you think Organovo will thrive in the future as they face profitability challenges and as more competitors enter the space. Additionally, do you foresee any additional regulatory scrutiny? I’m also interested to learn if you think that Organovo should specialize in liver production or if there are any additional benefits to expansion of 3D printing other types of human tissue?
Great article and interesting topic! You mentioned the issue of competition, which I also see as an issue in the 3D printing / bioprinting industry. How can Organovo (or others) create their own monopolies in this space? What do you think of open sourcing “designs” and allowing other companies to produce bioink, while maintaining access to those designs via Organovo exclusive? Is that a potentially good strategy?